Non-destructive characterization of collagen in normal and diseased tissue development
We have been developing imaging methods and analysis software to characterize collagen fiber organization for different biomedical applications (Bayan et al., 2009; Quinn and Georgakoudi, 2013, Liu et al. 2015, Liu et al. 2016). By utilizing non-invasive imaging techniques (e.g. two photon excited fluorescence, second harmonic generation, and confocal reflectance microscopy) and developing quantitative outcomes related to tissue composition and organization, we can provide unique insight into structural and mechanical changes during dynamic events that occur as tissues develop normally or when diseases, such as cancer, arise. The matlab code we have recently reported on to calculate 3D organization and variance of fiber like structures (Liu et al, 2015 and 2015) is available for download as a zip file.
Some specific projects in this area include:
Understanding the relationship between collagen fiber organization and local mechanical properties during mammary gland morphogenesis
Collaborators:
Ana Soto, MD, and Carlos Sonnenschein, MD, Cell, Molecular & Developmental Biology, Tufts Sackler School of Graduate Biomedical Sciences
In collaboration with Drs. Soto and Sonnenschein, we are investigating the relationships between extracellular matrix organization and cell morphology during 3D epithelial morphogenesis. In live cell microscopy studies, we have been exploring the effect of matrix composition on the morphology of developing epithelial breast cell structures and the surrounding collagen fiber organization. We are also studying the effects of hormone regulation on collagen fiber organization and the interactions between the cells and the collagen matrix. This work utilizes second harmonic generation and/or confocal reflectance microscopy to obtain non-destructive 3D maps of collagen fiber organization. We have also been investigating the effect of bisphenol-A (BPA), an estrogenic compound found in plastics, on mammary gland development in a mouse model. Exposure to environmentally-relevant BPA levels during fetal life increase the propensity to mammary cancer. We have specifically been investigating how exposure to BPA alters periductal fiber density and organization.
Related publications:
Speroni L, Whitt GS, Xylas J, Quinn KP, Jondeau-Cabaton A, Georgakoudi I, Sonnenschein C, Soto AM. Hormonal regulation of epithelial organization in a 3D breast tissue culture model. Tissue Engineering Part C Methods. In Press.
Quinn KP, Georgakoudi I (2013). Rapid quantification of pixel-wise fiber orientation data in micrographs. Journal of Biomedical Optics. 18(4): 046003.
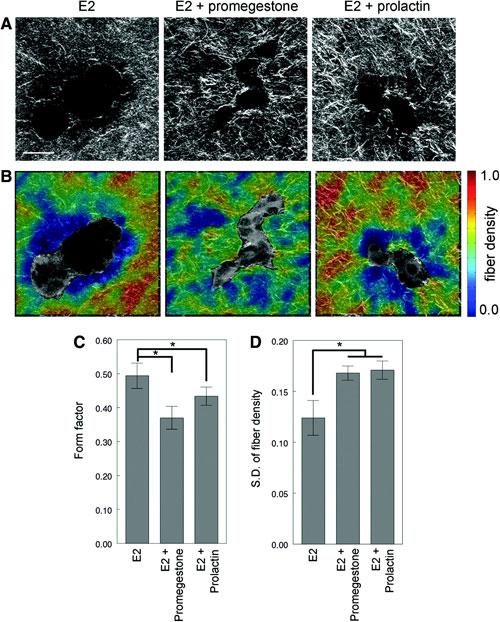
Figure 1. Collagen organization analysis of T47D cells growing in 3D cultures in the presence of β-estradiol (E2), E2+promegestone and E2+prolactin for 2 weeks. (A) Second harmonic generation (SHG) images, (B) fiber density maps on merged SHG and two-photon excited fluorescence channels, (C) form factor of epithelial structures, and (D) standard deviation of fiber density. Scale bar: 50µm. Adapted from Speroni et al., in press.
Characterizing structure-function relationships in decellularized cardiac tissues
Collaborator:
Lauren D. Black, PhD, Biomedical Engineering Department, Tufts University
In collaboration with the Black lab, we have been utilizing non-linear optical microscopy to characterize 3D ECM organization in decellularized heart tissue. The non-destructive nature of this imaging technique enables complementary mechanical testing and compositional analysis to elucidate relationships between our optical signals, tissue composition, and mechanical function. Currently, these techniques are being applied to projects studying heart development and the remodeling that occurs following a myocardial infarct.
Related publications:
Williams C, Quinn KP, Georgakoudi I, Black LD. Young developmental age cardiac extracellular matrix promotes the expansion of neonatal cardiomyocytes in vitro. Acta Biomaterialia. In press.
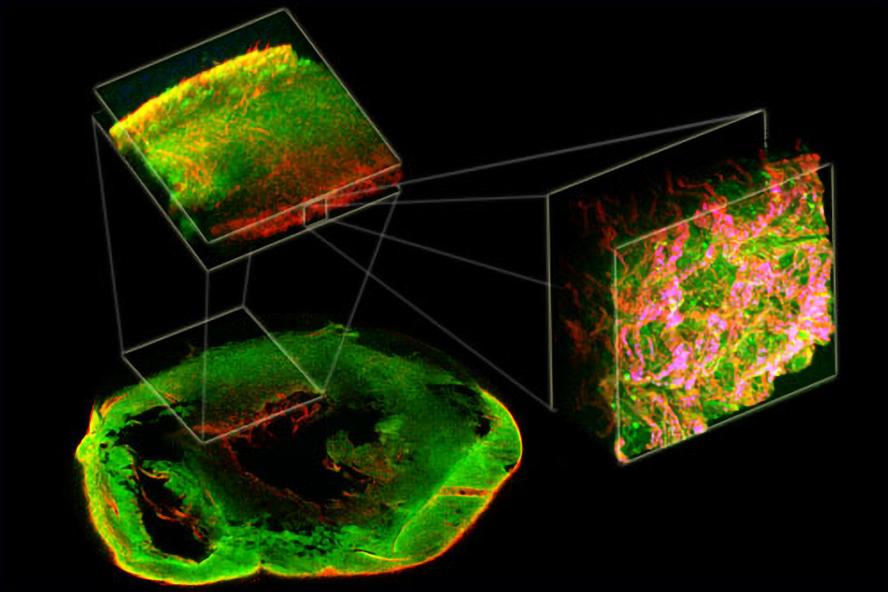
Figure 2. 1st Place VRTA illustration competition in 2012. Two-photon excited fluorescence (green) and second harmonic generation (red) from a decellularized rat heart demonstrates complex organization from the macro- to micro-scale.
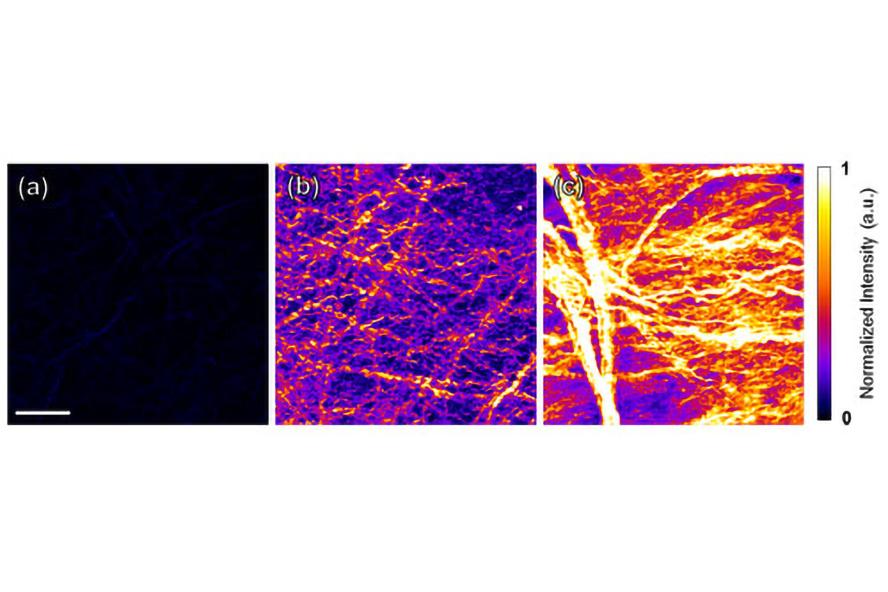
Figure 3. Second harmonic generation (SHG) images of decellularized (a) fetal, (b) neonatal, and (c) adult heart tissue. The increased SHG signal over time may be indicative of increased fibril size and organization within fiber bundles. Figure adapted from Williams et al., in press.
Movie 1. Two photon excited fluorescence (TPEF) of blood vessels (red) and second harmonic generation (SHG) of collagen (blue and green) within a decellularized heart image volume.
Assessing collagen fiber organization and composition during tendon development
Collaborator:
Catherine K. Kuo, PhD, Biomedical Engineering Department, Tufts University
We are currently exploring techniques to quantify changes in collagen fiber organization and crosslinking during tendon development using non-linear optical microscopy. Specifically, we are correlating quantitative optical markers with liquid chromatography / tandem mass spectrometry (LCMS) measurements of tendon composition using a embryonic animal models in collaboration with the Kuo lab. In addition, we are examining correlations between optical and biomechanical tissue properties as assessed by methods such as atomic force microscopy (AFM). By improving our understanding of these dynamic developmental processes through non-destructive 3D microscopy techniques, we ultimately aim to elucidate mechanical and biomechanical cues that can guide musculoskeletal regeneration strategies.
Related publications:
Marturano JE, Arena JD, Schiller ZA, Georgakoudi I, Kuo CK (2013). Characterization of mechanical and biochemical properties of developing embryonic tendon. Proc Natl Acad Sci U S A 110(16):6370-5. PMID: 23576745
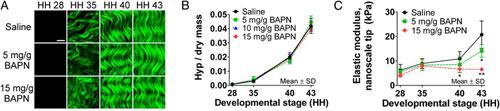
Figure 4. Monitoring collagen in developing chicken embryo tendons with β-aminopropionitrile (BAPN) treatment to inhibit collagen cross-linking. (A) Second harmonic generation (SHG) images showed no apparent differences in collagen microstructure with BAPN treatment compared with a saline control. (Scale bar, 10 μm.) (B) LCMS results also suggest that BAPN treatment had no effect on hydroxyproline (Hyp) content, representative of collagen content, during development (P ≥ 0.51; n = 3). (C) BAPN treatment significantly reduced tendon elastic modulus measured with a nanoscale AFM tip by 38% at HH 40 (*P < 0.05) and by 68% at HH 43 (**P < 0.001; n = 5). Figure adapted from Maturano et al, 2013.