Monitoring of circulating cells in vivo or within microfluidic devices
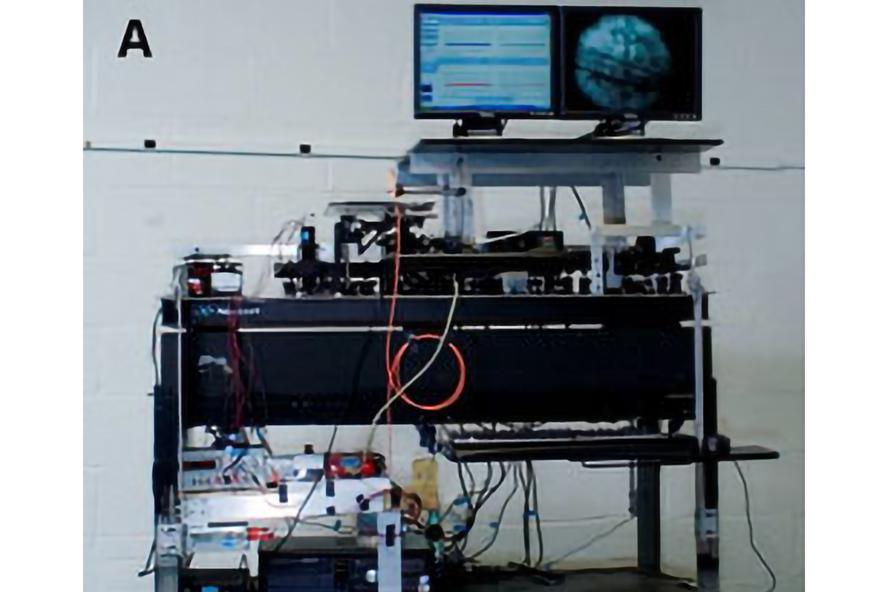
Picture of portable In Vivo Flow Cytometer
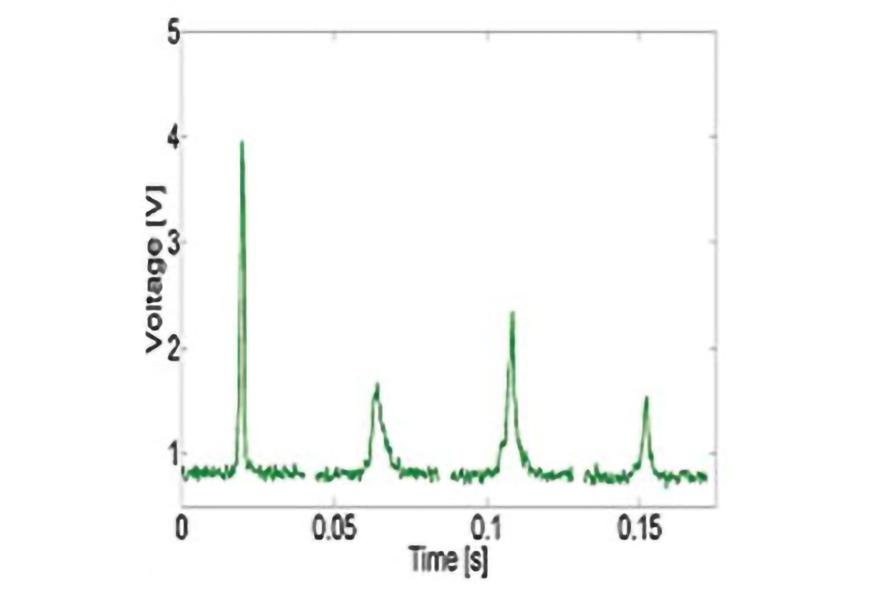
Example in vivo flow cytometry measurement of circulating GFP expressing cancer cells in mouse.
Sample image of blood vessels in mouse's ear. Excitation slit is typically aligned across the artery to monitor circulating cells of interest.
Flow Cytometry
The main goals of the project are:
- To monitor quantitatively the number of specific populations of circulating cells, such as white blood and cancer cells,
- To discriminate circulating tumor cells from white blood cells by exogenous fluorescence and endogenous light scattering intensity,
- To understand the role of circulating tumor cells during disease progression or treatment, and
- To further develop the approach for in vivo application in humans, i.e. where fluorescent tagging is not possible
Medical applications include non-invasive detection and monitoring of cancer patients.
In Vivo Flow Cytometry
In vivo flow cytometry, which combines confocal microscopy and flow cytometry, has been developed as a new non-invasive method of detecting and monitoring circulating cells. We use exogenous fluorescence and intrinsic scattering to discriminate among cell types. In the case of fluorescence detection, cells are tagged with fluorescent dyes conjugated to antibodies specific to white blood cells or tumor specific markers. Alternatively, the cells may be transfected to express a fluorescent protein. As labeled cells flow naturally in the blood, the cells are excited as they traverse one or more laser beams in single file. Using multiple incident wavelengths, fluorescence and scattered photons are collected by detectors. A confocal slit by the detector eliminates background fluorescence, thereby improving the signal to noise ratio.
Current research is focused on the detection and counting of two clinically important cell populations: leukocytes and tumor cells. The number of white blood cells in circulation can indicate the progression of disease as well as the efficacy of treatment; their numbers are typically monitored during infection, organ and bone marrow transplants, chemotherapy and AIDS. In vivo flow cytometry could allow for blood cell analysis without the need to take blood samples. Moreover, since the abundance of circulating tumor cells is of prognostic values, we address fundamental questions related to the role of these cells during cancer progression and metastasis. One of our recent studies correlated the number of GFP-expressing circulating tumor cells and the concentration of red autofluorescent white blood cells to the extent of metastases in mice.
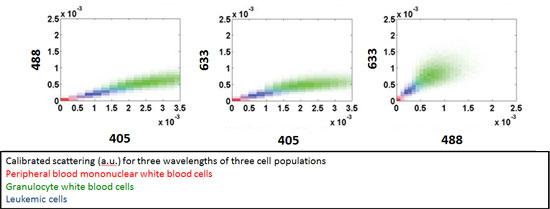
Beyond fluorescence-based detection, our efforts have focused on discriminating circulating cancer and tumor cells from white blood cells within blood samples. Differences in cell morphology and granularity, as measured by backscattered light intensity, are the bases for quantifying the circulating cancer cell burden. We use a custom-built flow cytometer equipped with three lasers of different wavelengths. A study comparing leukemic and normal white blood cells showed that leukemic cells occupy an intermediate scattering space in between mononuclear and granulocyte white blood cells, as seen in the 2-D scatter plots below (Greiner at al. 2011, Hsiao et al. 2011) . Our current work expands this detection platform into circulating tumor cells derived from solid tumors, such as those found in epithelial breast tissue.
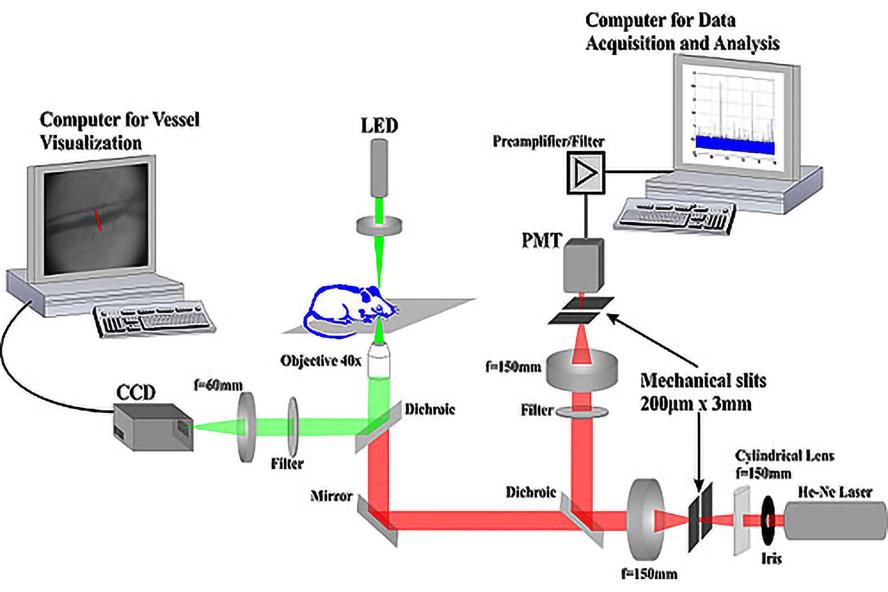
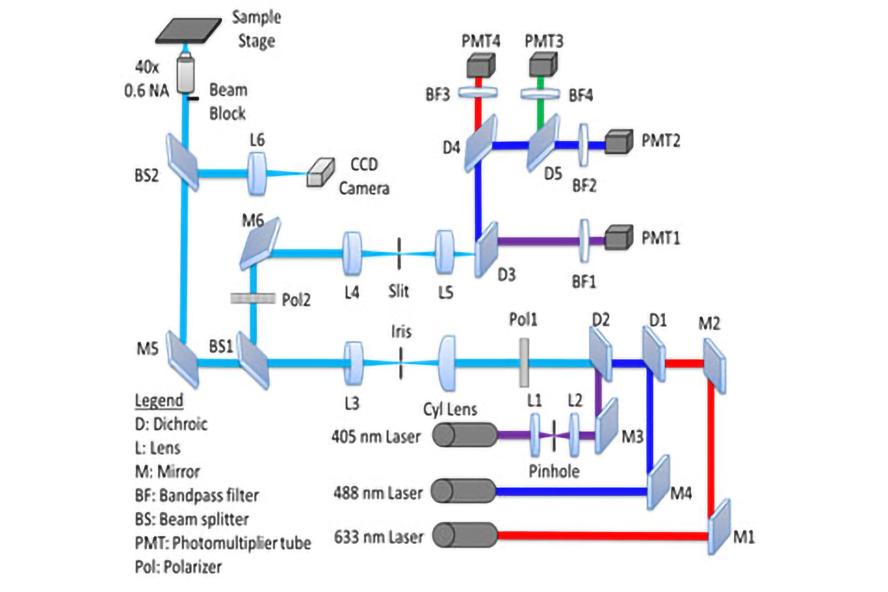
Optical schematic of In Vivo Flow Cytometer
Custom flow cytometer optical design, microfluidic channel parameters, and slit illumination specifications. Key components of the optical system include parallel polarization, fluorescence detection, beam block, and tilted slit illumination.
Collaborators:
Rachel Buchsbaum, Tufts University School of Medicine, Molecular Oncology Research Institute
Charlotte Kuperwasser, Tufts University School of Medicine, Developmental, Molecular, and Chemical Biology
Gail Sonenshein, Tufts University School of Medicine, Developmental, Molecular, and Chemical Biology