Bioelectronic scaffolding with silk fibroin
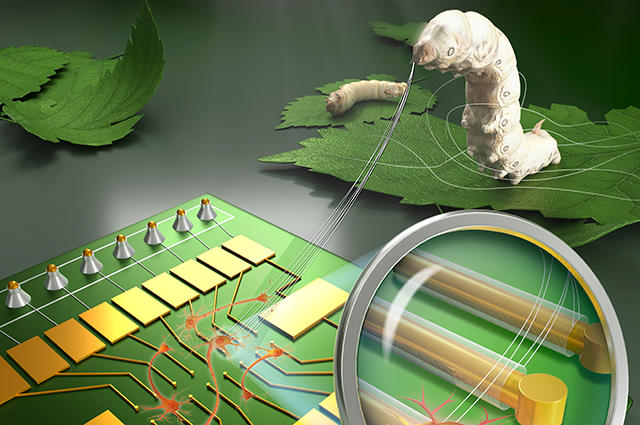
Recent developments in bioelectronics have allowed researchers to engineer hybrid tissues with embedded devices that monitor, modulate, or augment cellular function. These hybrid tissues must not only incorporate bioelectronic devices but also electrical interconnects that allow the devices to be addressed. This challenge may be approached with highly flexible and porous bioelectronic scaffolds which can be fabricated with conventional photolithography processes and, when integrated with tissues, cause minimal mechanical disruptions. One common material used for this purpose is SU8, a photo-crossable epoxy that both supports devices and provides electrical passivation to the interconnects. While this material is effective, an alternative bioactive polymer has the potential to promote cellular adhesion to achieve a more seamless integration between scaffolds and tissues.
Assistant Professor Brian Timko and colleagues have demonstrated that a photo-cross-linkable silk fibroin (PSF), derived from the cocoon of Bombyx mori, may serve as an ideal bioelectronic integration scaffold due to the material’s structural, electrical, and chemical properties. The research, published in Proceedings of the National Academy of Sciences of the United States, characterizes the promising properties of PSF bioelectronic devices.
PSF displayed an ability to be shaped into high-resolution, three-dimensional constructs using conventional photolithography techniques. PSF films produced in the study offered tunable thickness and a spatial resolution of less than one micrometer. These properties make PSF suitable for bioelectronic applications in which stable, detailed structures are desired.
The team fabricated microscale bioelectronic recording elements that were addressed by interconnects passivated with PSF. These devices provided stable bioelectronic readouts from both cardiac and brain tissue models for at least 8 days. They provided these signals with extremely high signal-to-noise rivaling that achievable with SU-8 based devices. These results are attributed to the material’s relative stability and its natural electrical insulation.
The researchers also found the material to have an amendable surface chemistry, which allows for chemical customization through reactive amino acid side-chain groups. By coating the scaffolds with poly-lysine, an adhesion factor commonly used in bioelectronics, the team was able to promote greater cellular adhesion compared to similarly-treated SU8. Cellular adhesion was particularly enhanced when the material was tested with human induced neural stem cells (hiNSCs), which have been used in 3D brain tissue models.
Timko said, “While PSF exhibited interesting electrical and mechanical properties, we were especially excited when we saw how well it adhered hiNSCs. In bioelectronics it is critical to obtain a tight junction between living and synthetic materials, and our PSF represents a significant advance in that area. Our next step is to integrate these materials into engineered tissues, for brain models, to determine if we can achieve long-term bioelectronic readouts.”
The team’s findings demonstrate that PSF is a promising route towards not only brain models but also a wide variety of cell and tissue types. The properties described in the research demonstrate PSF’s ability to facilitate new classes of hybrid tissues with seamlessly integrated bioelectronics—or tissue “cyborgs.”
Lead author on the study was Dr. Jie Ju, now an Assistant Professor at Henan University, China. Co-authors are Drs. Ning Hu, Dana Cairns and Haitao Liu.
Read more in Proceedings of the National Academy of Sciences of the United States: Photo–cross-linkable, insulating silk fibroin for bioelectronics with enhanced cell affinity.
Department:
Biomedical Engineering