Multiple paths to enhance PAL activity
With an array of possible agricultural and medicinal applications, the class of enzymes called phenylalanine ammonia-lyase (PAL) holds a great deal of potential, including as a therapeutic enzyme in cancer treatment or in the treatment of phenylketonuria (PKU), an inborn error of metabolism that can lead to seizures, behavioral problems, intellectual disability, and mental disorders. The PAL from Anabaena variabilis (Trichormus variabilis), in particular, is the active ingredient in Pegvaliase, the only drug approved by the U.S. Food and Drug Administration (FDA) to treat classical PKU.
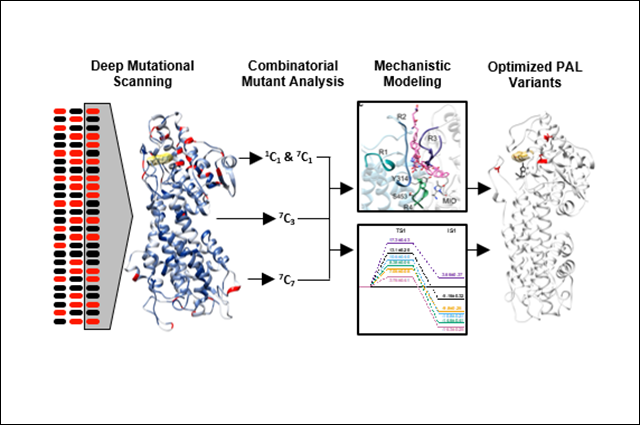
Researchers’ ability to engineer these enzymes is currently limited because the protein-wide sequence determinants of function are unknown, despite the availability of extensive research on catalytic mechanism, structure, and substrate specificity. A team of researchers from Tufts’ Synthetic Biology and Systems Bioengineering Lab – led by Associate Professor Nikhil Nair of the Department of Chemical and Biological Engineering – and from Kcat Enzymatic Private Limited, an India-based enzyme/protein engineering group, took up the problem.
In research reported in the journal ACS Catalysis, the team leveraged previous work to perform deep mutational scanning and was able to create a detailed sequence-function landscape of PAL. They found 79 hotspots that positively affected enzyme fitness – many of which had not previously been discovered or reported – and continued to further the study of PAL to understand how mutations alter the energetics of the reaction.
The team consisted of first author and postdoctoral scholar Vikas Trivedi, alum Todd Chappell, EG20, former postdoctoral scholar Karishma Mohan, alum Athreya Ramesh, E21, and corresponding author Nair, working with colleagues Naveen Krishna, Anuj Shetty, Gladstone Sigamani, and Pravin Kumar of Kcat Enzymatic Private Limited. Chappell, Trivedi, and Nair have spun-out Enrich Bio, Inc., to translate the engineering method and enzyme variants into the clinic to for improved treatment options for PKU and other similar metabolic disorders.
Department:
Chemical and Biological Engineering