Improving the efficacy of therapeutic cancer vaccines
Over the past several decades, researchers have worked to develop vaccines that support the treatment of cancer. There are currently two FDA-approved vaccines that prevent cancer: the HPV vaccine and the Hepatitis B vaccine. However, only one dendritic cell-based vaccine (Sipuleucel-T) has been approved by the FDA to treat existing cancer (others are still in clinical trials) and the overall clinical benefit of immunotherapeutic cancer vaccination is low.
There are two major hurdles slowing the advancement of these cancer vaccines: the high variability of tumor associated antigens (TAAs) and the immunosuppressive tumor microenvironment.
Immunotherapeutic cancer treatment works by boosting the body’s immune response to antigens. Antigens are substances found on the surfaces of cells that the immune system typically identifies as harmful. Cancer can occur when the immune system no longer naturally recognizes these antigens as harmful. Immunotherapeutic vaccines work to retrain the immune system to fight specific antigens with anti-tumor T cells. These T cells help destroy harmful cells and generate a “memory” that the immune system can later recall as needed.
In practice, antigens produced by tumors are highly variable and the complexities associated with developing personalized vaccines means that treatment is only available to a small portion of the population. Additionally, cancer patients have weakened immune systems and often do not produce strong immune cell responses to these vaccines.
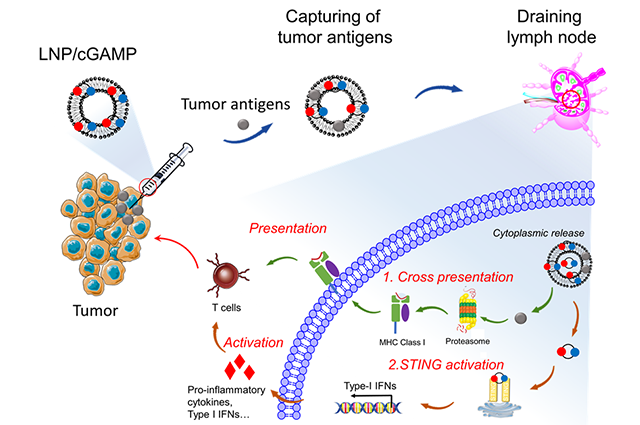
Researchers led by first author and postdoc associate Dr. Jinjin Chen and Associate Professor Qiaobing Xu of the Department of Biomedical Engineering have set out to address these issues by designing a lipidoid nanoparticle (LNP), labeled 93-O17S-F/cGAMP, that can be administered in situ, or locally, to enhance the presentation of tumor antigens and support intercellular delivery to immune cells.
The LNP system developed by the team works by first injecting a tumor with a small dose of doxorubicin, a chemotherapy medication. This injection induces tumor immunogenetic death and the release of the TAAs. Meanwhile, the team loads the LNP with cGAMP, an agonist which, when introduced within the tumor, triggers an immune response called the STING pathway. This pathway plays an important role in the production of cytotoxic T cells for killing cancer cells.
After pre-treatment is complete, the LNP/cGAMP is injected near the tumor, where it is hypothesized that it captures the previously released antigens via electrostatic interaction. The LNP/cGAMP captures TAAs and then delivers them to draining lymph nodes (DLNs). Once within the antigen presenting cells (APC) in DLNs, the LNP releases its cargo intracellularly, which triggers the cross-presentation of TAAs and activation of cytotoxic T cells response via STING pathway.
The resulting treatment is promising due to its ability to activate a cytotoxic T cell response within the tumor microenvironment, reverse the immunosuppressive tendencies of the tumor, and harness variable antigens without the need for personalized treatment.
The team found that 93-O17S-F/cGAMP, in combination with a pre-treatment of doxorubicin, demonstrated antitumor efficacy up to 35% of mice exhibiting total recovery from a primary tumor. Moreover, 71% of mice demonstrated a complete recovery from a subsequent challenge. These results indicate an immune memory against the tumor, providing a promising strategy for in situ cancer vaccination that should be investigated further.
Read “In situ cancer vaccination using lipidoid nanoparticles” in Science Advances.
Department:
Biomedical Engineering